Malaysia Medical Device Authority: Full Implementation of Registration Requirements (1st January 2018)
The Malaysia Medical Device Authority issued a notice stating that, as of 1st of January 2018, the agency will fully enforce the medical device registration requirements as specified under Section 5 of the Medical Device Act 2012 (Act 737)On the 25th of July 2017, the Malaysia Medical Device Authority (MDA) under the Ministry of Health issued a notice stating that, from 1st of January 2018, the agency will fully enforce the medical device registration requirements as specified under section 5 of the Medical Device Act 2012 (Act 737) before importing, exporting or placing medical devices in the Malaysian market.
From that date onwards, all medical devices to be imported, exported or placed in the Malaysian market will need to meet the licensing and other requirements as set out in Section 5 of the Medical Device Act 2012 (Act 737). We advise any companies that have applied for the registration of medical devices under this Act, but have yet to submit all the information required, to complete the application on or before 31st of October 2017.
The announcement also stated that,as of 1st of January 2018, only the license establishment certificate and the medical device registration certificate are acceptable as supporting documents in the procurement process of medical devices in any health facility institution. The letter, “Acknowledgment receipt of application for medical device registration under Medical Device Act 2012 (Act 737)”, is no longer valid for use as a supporting document.
From that date onwards, all medical devices to be imported, exported or placed in the Malaysian market will need to meet the licensing and other requirements as set out in Section 5 of the Medical Device Act 2012 (Act 737). We advise any companies that have applied for the registration of medical devices under this Act, but have yet to submit all the information required, to complete the application on or before 31st of October 2017.
The announcement also stated that,as of 1st of January 2018, only the license establishment certificate and the medical device registration certificate are acceptable as supporting documents in the procurement process of medical devices in any health facility institution. The letter, “Acknowledgment receipt of application for medical device registration under Medical Device Act 2012 (Act 737)”, is no longer valid for use as a supporting document.
Company activities 2019Company activities 2015 |
EECP® | PERSPECTIVE OF A CARDIOTHORACIC SURGEON BY DR. LIM YEW CHENG
EECP® PATIENT SELECTION : Guidelines for safe screening of EECP® Patients in outpatient GP Setting Workshop
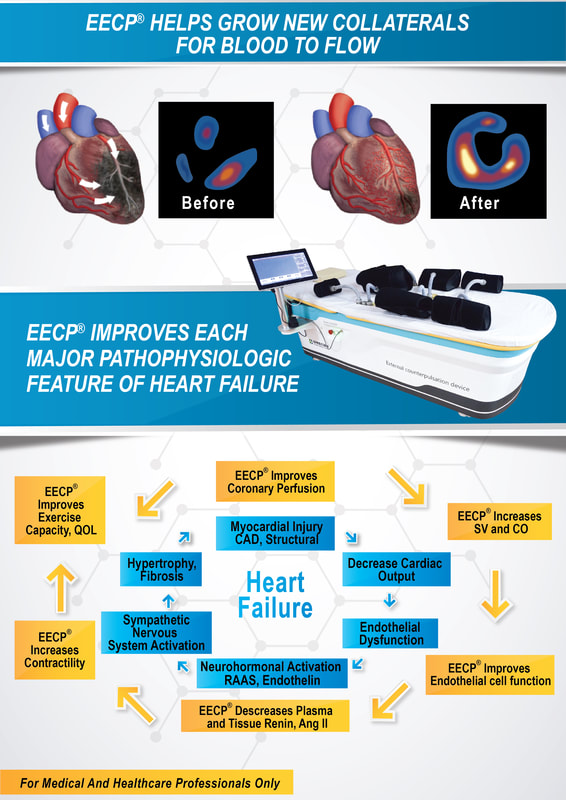
EECP® Therapy - A simple, non-invasive solution to the complex problem of angina and heart failure.Offered exclusively by MPCN dot com Sdn Bhd (establishment licence KP6302271315) , EECP® Therapy is a safe, non-invasive, outpatient treatment option for patients suffering from ischemic heart diseases such as angina and heart failure.
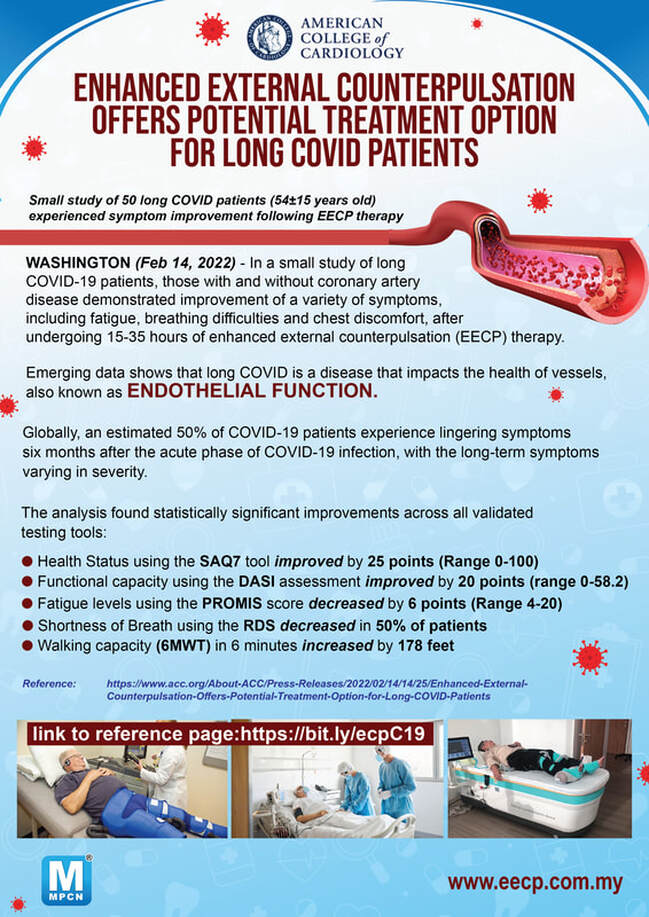
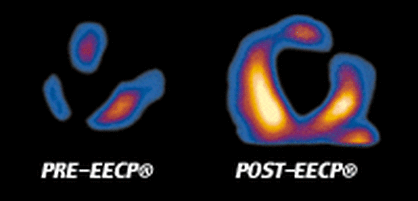
EECP® Therapy has helped hundreds of thousands of patients around the world. Clinical studies show over 75% of patients benefit from EECP® Therapy with sustained improvement up to 3 years post-treatment. Slideshow - Quick Glance of EECP® Studies |
What is EECP® Therapy?For people with angina or heart failure, even simple activities - such as going to the mailbox or walking the dog - can be challenging. If you are one of these people, take heart. There is a non-invasive treatment called EECP® Therapy that clinical experience has shown to be safe and to have benefit for the treatment of angina and heart failure. Approximately 80% of patients who complete the 35-hour course of EECP® Therapy experience significant symptom relief that may last up to three years. EECP® Therapy is an outpatient treatment for angina and heart failure. Treatments are usually given for an hour each day, five days a week, for a total of 35 hours. During the treatment, you lie on a comfortable treatment table with large blood pressure-like cuffs wrapped around your legs and buttocks. These cuffs inflate and deflate at specific times between your heartbeats. A continuous electro cardiogram (ECG) is used to set the timing so the cuffs inflate while the heart is at rest, when it normally gets its supply of blood and oxygen. The cuffs deflate at the end of that rest period, just before the next heart beat. The special sensor applied to your finger checks the oxygen level in your blood and monitors the pressure waves created by the cuff inflations and deflations. |
|
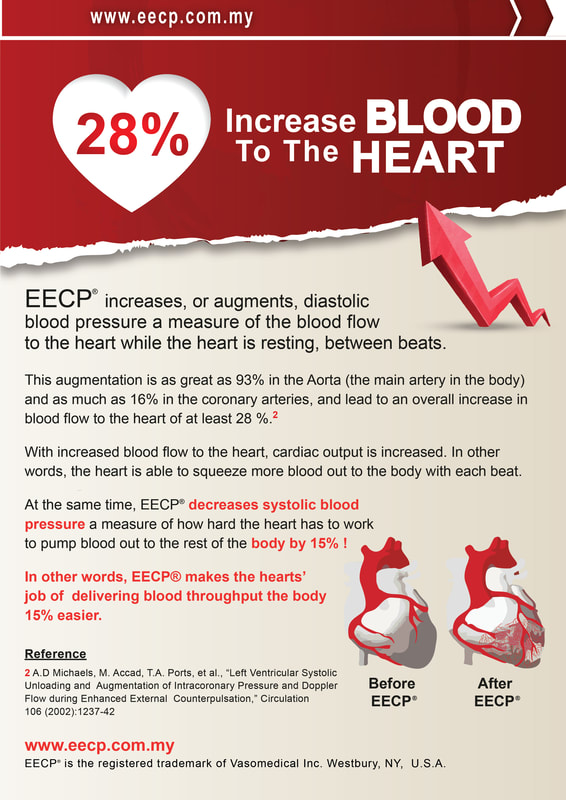
AdvantagesEECP® (Enhanced External Counterpulsation) Therapy and ECP (External Counterpulsation) Therapy are the terms which describe the non-invasive, complimentary or alternative treatment prescribed by physicians for patients suffering from the symptoms of angina, congestive heart failure and other cardiovascular diseases. Both EECP Therapy, a name which has been trademarked by Vasomedical Inc., Westbury, NY in the marketing of their treatment method and treatment devices they manufacturer and ECP Therapy, the generic term used to describe all other forms of external counterpulsation (including the ECP systems manufactured by PSK – Chongqing, China) , are based upon the proven hemodynamic principal of counterpulsation, which involves increasing blood flow (volume and pressure) to the heart during diastole (when the heart is at rest) and reducing the workload and oxygen demand the heart uses to pump blood throughout the body in systole.
In addition to the clinically proven hemodynamic principal of counterpulsation, extensive research in clinical studies published in peer-reviewed medical journals have demonstrated the many mechanisms of action that are responsible for the initial benefits produced by the therapy as well as the long term outcomes, which show these initial benefits lasting three to five years. While every year a number of clinical articles on EECP and ECP continue to be published in medical journals and textbooks worldwide, some using PSK ECP systems and supported by PSK, most of the over 200 clinical articles published in the literature on EECP and ECP therapy were performed on Vasomedical EECP therapy systems and reported in the clinical research supported by Vasomedical. |
Decisive advantage at a glance:EECP®, is a registered trademark of Vasomedical and may only be used to describe the external counterpulsation therapy delivered by using a Vasomedical EECP® Therapy system. EECP® therapy has been clinically proven to be the highest standard of external counterpulsation. There are over 200 publications in medical journals, many of them peer-reviewed, demonstrating the clinically efficacy of EECP® therapy. This is the gold standard of clinical proof, in external counterpulsation.
The successful application of external counterpulsation involves several key elements:
|
|
|
|
|
|
|
EECP® SAFE
|
EECP® from physiology to clinical practice - Speaker : Professor Gregory W Barsness from Mayo Clinic-USA |
EECP® -The Last Resort for Refractive Angina- Speaker
|
EECP® in treatment
|
Take Action now
|