HB-AECP® TOUCHSCREEN
Technical Parameters & Specification
Scientific Name : Enhanced External Counterpulsation Device
Item No : HB-AECP®
Material : Metal Frame for Main Support
Volume : 2.5m3
Voltage : Single exchange 110V - 220V 50/60 Hz
Power Supply : ≤ 2600W
Dimension : L*W*H = 2010*780*700 (mm)
Net Weight : 200 KG
Scientific Name : Enhanced External Counterpulsation Device
Item No : HB-AECP®
Material : Metal Frame for Main Support
Volume : 2.5m3
Voltage : Single exchange 110V - 220V 50/60 Hz
Power Supply : ≤ 2600W
Dimension : L*W*H = 2010*780*700 (mm)
Net Weight : 200 KG
|
Contraindications
Enhanced ECP Therapy should be used for the treatment of patients with:
Our Advantage
clinical experience. 3. Top Key Parts
4. HB-AECP have Accurate & Sensitive Response And Treatment for the premature patients. Full inflation and deflation when working, clinic result is better for patients. |
Precautions
Decreasing cardiac afterload by optimizing diastolic augmentation may help minimize increased cardiac filling pressure due to venous return.
Certain valve conditions, such as significant aortic insufficiency, or severe mitral or aortic stenosis, may prevent the patient from obtaining benefit from diastolic augmentation and reduced cardiac afterload in the presence of increased venous return. |
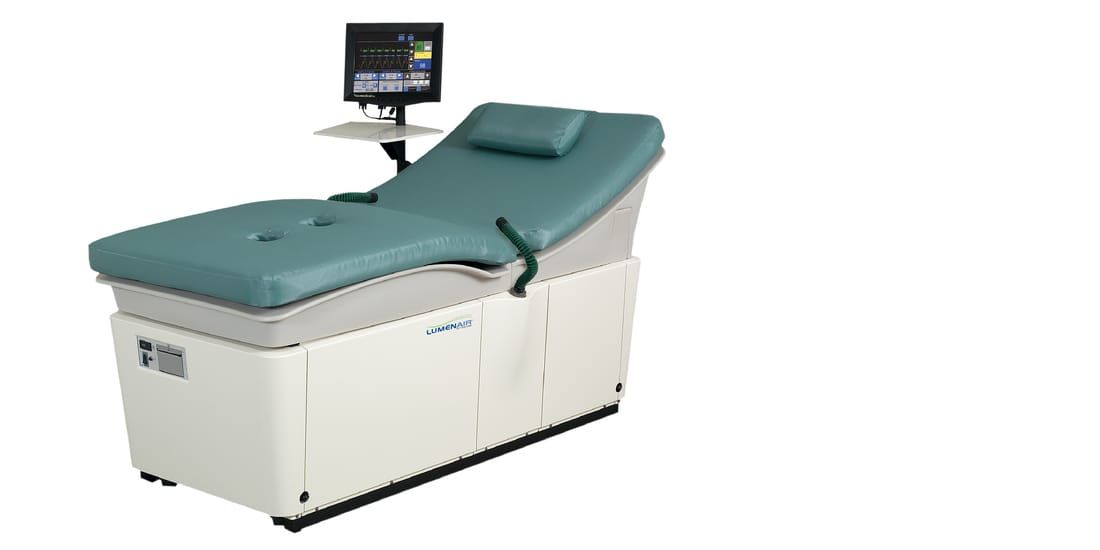
Vasomedical LumenairECG Amplifier
Input impedance : >2 MΩ Common mode rejection ratio : >90 dB Bandwidth : 1-20 Hz on display Sp0₂ Oxygen Saturation Oxygen saturation range : 0 to 100% Accuracy Sp0₂ (± 1 Standard Deviation) 70-100% ±2 digits for adults using the Finger Clip Sensor 70-100% ±3 digits for adults using Flex or Reflectance Sensors 70-100% ±2 digits for adults using Ear Clip Sensor Below70% is not specified for all sensors Triggering Method External trigger : R-wave of patient ECG External trigger ratio : 1:1 or 1:2 ECG trigger range : 35-125 ±1 bpm Filter Technique Low-pass digital filter eliminates 50/60 Hz and high-frequency interference. Treatment Pressure Range Adjustable from 80-300 mmHg Cuffs Each set includes calf, lower thigh and upper thigh/buttock cuffs with quick disconnects. Table Safe Working Load 400 Ib (180 kg) Treatment Session Timer Set treatment time up to 60 minutes maximum. System automatically stops when set time expires. System Protection Three circuit breakers - Mains and compressor, and a built-in circuit breaker in the power switch on the front panel |
Patient Protection / Safety
Equipment Dimensions and Weight Height : 42” (107cm) Width : 34” (87cm) Length : 78.5” (200cm) Weight : 550 Ib (250kg) Operating Voltage Requirement (switch selectable) 115VAC, 50/60 Hz, 20A 230VAC, 50/60 Hz, 15A Operating Environment Temperature : 50 to 104°F (10 to 40°C) Relative Humidity : 30to 75% (no condensation) Atmospheric Pressure : 700 to 1060 hPa Atmosphere : Free of corrosive gas Transport and Storage Environment Temperature : 14 to 140°F (-10 to 60°C) Relative Humidity : 30 to 85% (no condensation) Atmospheric Pressure : 500 to 1060 hPa Atmosphere : Free of corrosive gas |
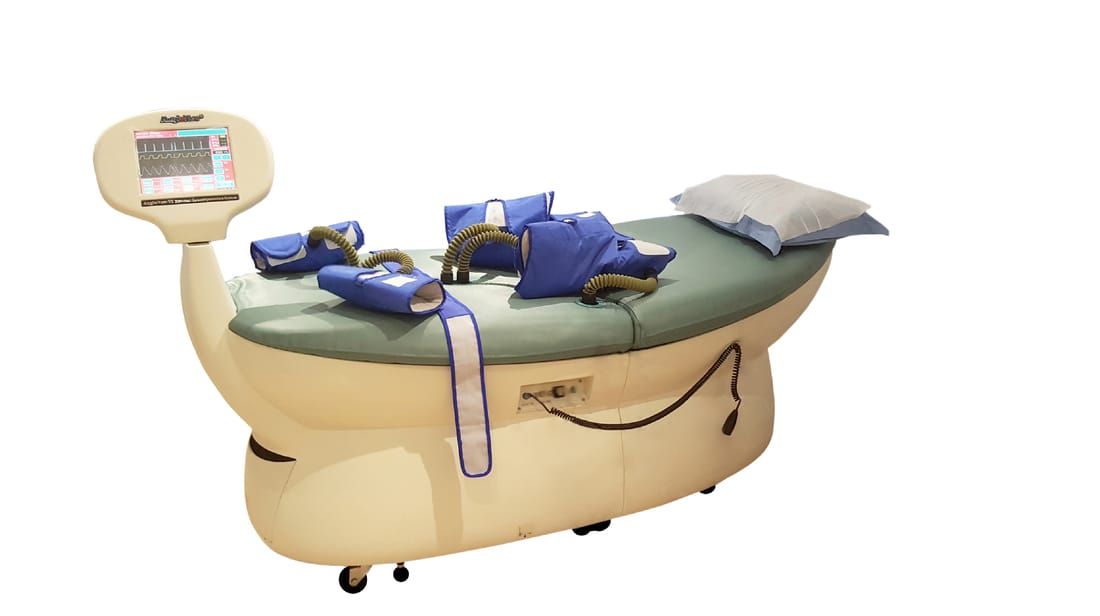
AngioNew VI
|
SYSTEM & SAFETY FEATURE
System features promote safe, comfortable and effective patient treatment :
|
SYSTEM SPECIFICATIONS
Operating Voltage
|